Contract number
J1-9162
Department:
Department of Biology
Type of project
ARIS projects
Type of project
Basic research project
Role
Lead
Financing
Duration
01.07.2018 - 30.06.2021
Total
1.50 FTE
Project manager at BF
Drobne DamjanaABSTRACT
INTRODUCTION: One of the most promising applications of nanoscience in medicine is in the diagnosis, treatment and prevention of Alzheimer’s disease (AD), but nanomaterials have also been attributed as a causative factor of neurodegenerative diseases. Therefore, current and future dynamics of nanotechnology will be based on the balance of these two realities.
Alzheimer’s dementia and aging populations are a global priority for research and health policy. In Slovenia, this area is placed within Strategic Research and Innovation Partnerships (SRIP) Health-Medicine. The lead partner of this proposal, prof. dr. Damjana Drobne, is coordinating pillar “Active and healthy aging” within this strategic area where priority is given to neurodegeneration (http://www.sis-egiz.eu/en/activities/srip/).
Despite a lot of effort, the basic cause of AD is still not entirely clear and presently no cure exists that could reverse neurodegeneration. Consequently, research in the field of neuroprotection is gaining importance. In this context, products of nanotechnologies could be used as carriers of antioxidants and cholinesterase inhibitors, assist in biomarker development to aid in early neurodegeneration diagnostic tools and for the development of new neurodegeneration treatments.
AIM: The aim of this project is to provide basic knowledge to support the application of nanomaterials for their beneficial use in neuroprotection, neurotoxicity prevention and in the early diagnosis of neurodegeneration.
Materials and methods: In our work we will address the diagnosis and prevention of neurodegenerative disease. The project presented here is organised into three interconnected phases looking at nanomaterials neurotoxic and neuroprotective effects in both in vivo and in vitro models. In the test systems applied, interactions between nanomaterials and neuronal and endothelial cells in vitro (Phase I) as well as in in vivo (Phase II) will be studied. In Phase III, the identified biomarkers will be validated with samples from patients with neurodegenerative disorders. As the final outcome of the project, high throughput nanomaterial protein corona fingerprint test will be elaborated.
Selected candidate biomarkers related to neurotoxicity/neurodegeneration are: (a) composition and abundance of cell-derived vesicles (exosomes) and (b) alterations in acetylcholinesterase amounts, splice variants and activity.
DISCUSSION: We believe that the results generated in the proposed project will assist in the selection of nanomaterials by highlighting those with neurotoxic potential and identifying those which support neuroprotection. Biomarker development and validation is designed to aid in the early diagnosis of neurodegeneration. A novelty in our study will be the use of a (c) nanomaterial serum protein corona fingerprint approach to compare blood samples of healthy individuals and AD patients. The proof of concept of nanomaterial protein corona fingerprint test was developed in our previous study (Sopotnik et al. 2015).
CONSORTIUM: The lead partner of the project is a member of consortia submitting application for COST action entitled The European Network for Alzheimer’s Disease, Acronym: EUNAD (Open Call Collection OC-2017-1, Proposal Reference OC-2017-1-22432). The COST action consortium posits that AD begins as a chronic injury in the cerebral vasculature and has an earlier origin in the heart and vascular system, not the brain. For the purpose of this project, lead partner prof. dr. Damjana Drobne organized a consortium of experts from different fields who have previously collaborated and have proven scientific excellence in the field of cell biology and microscopy (UL MF), biophysics of membranes (UL FE), health sciences (UL FHS), biochemistry and molecular biology (IJS), and analytical chemistry and laser spectroscopy (UNG). The lead partner has expertise in bio-nano interactions, nanotoxicology, biomarker development and cholinesterases.
THE PHASES OF THE PROJECT AND THEIR REALIZATION
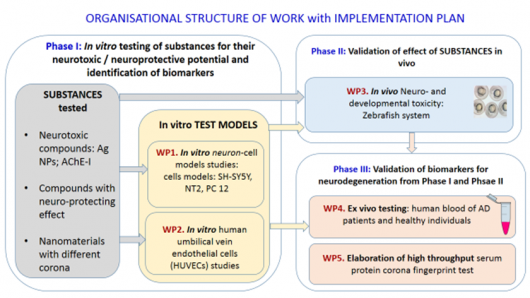
The project is divided into three interconnected phases. Those are composed of work packages (Figure 1).
Figure 1. Detailed organisational work packages (WPs) and implementation plan. Arrows indicate interconnection between Phases and WPs.
The project will be divided into work packages (WPs) (Table 1).
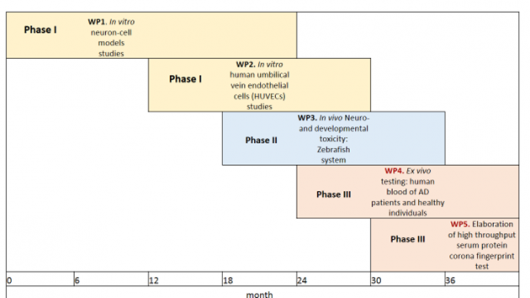
Table 1. Timetable of the project with phases and workpackages (WPs).
- WP1: In vitro neuron-cell models studies: cells models: SH-SY5Y, NT2, PC 12 (UL BF, UL FE, UNG).
- WP2: In vitro human umbilical vein endothelial cells (HUVECs) studies (UL BF, UL MF, IJS).
- WP3: In vivo Neuro- and developmental toxicity: Zebrafish system.
- WP4: Ex vivo testing: human blood of AD patients and healthy individuals (UL BF, UL FHS).
- WP5: Elaboration of high throughput serum protein corona fingerprint test for simplified neurotoxicity evaluation and evaluation of substances with neuroprotective potential (all partners).
Project starts with in vitro testing (Phase I) and will continue with in vivo and ex vivo studies. The phases have one or two WPs and one or two milestones which are needed to be reached before starting the next phase (Figure 2). The most important milestones include Identification of biomarkers for neurotoxic substances in vitro on neuronal and endothelial cells, results on in vitro effect of neurotoxic/neuroprotective effect substances and effect of NMs with corona and finally validation of biomarkers for neurotoxicity identified in in phase I and phase II. Latter will be the basis for most important deliverable of the project: operational procedure for high throughput serum protein corona fingerprint test.
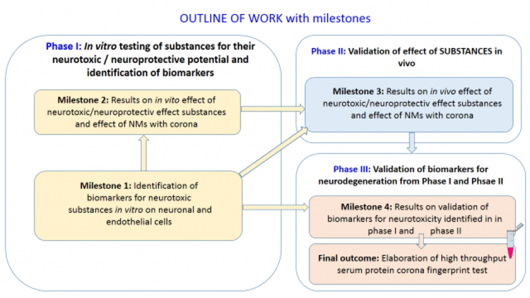
Figure 2. Outline of work with milestones which are interconnected.